History
The Lotus flower (Nelumbo nucifera) is the symbol of purity in several Asian religions. The major reason for this might be the self-cleansing property of its leaves. Even after emerging from mud, the leaves do not retain dirt when they unfold. This property has been studied intensively by the two botanists Barthlott and Neinhuis from the University of Bonn. In 1975 they discover the reason for this self-cleansing effect. Before that it was the general opinion that the smoother a surface, the less dirt and water adhere to it. By using a scanning electron microscope (SEM) the two scientists discovered that the surface of some Lotus leaves was everything else but smooth. It showed a combination of nano- and microstructures that gave the surface a rough structure. The explanation for the effect lies in two physical characteristics: the properties of these microstructures repel water and the nanostructures found on top of the microstructures are made of waxy materials which are badly wettable. The combination of the chemistry, the ultra structures, and the adherence properties of dirt and water to the surface, is what Barthlott and Neinhuis named the Lotus-Effect.
The Physical Basis
To understand the physics behind the Lotus-Effect, one has to take a look the forces that act upon a drop of liquid on a surface.
Forces acting on a liquid droplet on a solid surface
The shape of a drop on a surface is determined by three forces pulling at the three-phase contact line, the line between the solid and the liquid phase (S,L), between the solid and the vapor phase (S,V), and between the liquid and the vapor phase (L,V). All three forces together define the overall surface tension (energy per unit surface). The contact angle Y depends directly on the three surface tensions : The roughness of a surface improves the wettability for hydrophilic surfaces (Y < 90°). The drop will seem to sink into the hydrophilic surface. The wettability decreases for hydrophobic surfaces (Y > 90°). It gets energetically too expensive to wet a rough hydrophobic surface. The result is an increased water-repellency. Energetically the best configuration for the drop is on top of the corrugation like “a fakir on a bed of nails”.

A droplet on a hydrophilic rough surface seems to sink into the gaps

A droplet on a rough hydrophobic surface sitting on the spikes
Self-Cleaning Properties
A droplet on an inclined superhydrophobic surface does not slide off; it rolls off. When the droplet rolls over a contamination, the particle is removed from the surface if the force of absorption of the particle is higher than the static friction force between the particle and the surface. Usually the force needed to remove a particle is very low due to the minimized contact area between the particle and the surface. As a result, the droplet cleans the leaf by rolling off the surface.
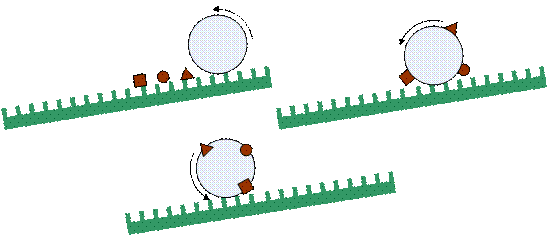
Diagram showing a droplet cleaning a superhydrophobic surface by rolling off




 A Szegedi Füvészkertért Alapítvány
A Szegedi Füvészkertért Alapítvány Rendezvényeinkről, fejlesztéseinkről rendszeresen hírt ad a media. Ezeket gyűjtjük itt össze…
Rendezvényeinkről, fejlesztéseinkről rendszeresen hírt ad a media. Ezeket gyűjtjük itt össze… Rendezvényeinkről, ovashat a linkre kattintva
Rendezvényeinkről, ovashat a linkre kattintva Nem töröltük régebbi cikkeinket sem, nézzen szét archívumunkban.
Nem töröltük régebbi cikkeinket sem, nézzen szét archívumunkban.